Proof vs. Progress: Why Quality Teams Struggle to Improve

It’s audit week. Again.
Your team has spent the last ten days digging up certificates, cross-checking expiry dates, and uploading the same evidence into three different portals. The folders are neat, the documentation is spotless, and every box on the checklist is ticked.
By Friday afternoon, the auditor signs off with a polite nod — “Well done, everything looks in order.”
It should feel like a win. But instead, there’s a quiet sense of frustration. Because deep down, everyone knows the same routine will repeat itself in a few weeks — new questionnaires, slightly different formats, identical information.
For many quality professionals, compliance has become a full-time occupation, leaving little time for the work that actually improves processes. Root cause analyses get postponed. Preventive measures remain ideas on a whiteboard. And “continuous improvement” starts to feel like a slogan rather than a system.
The irony is clear: the more effort teams invest in proving compliance, the less capacity they have to make meaningful progress.
2. Proof vs. Progress — The Two Sides of Quality
Every quality team juggles two types of work: the work that proves compliance, and the work that drives improvement. Both matter — but they don’t carry equal weight in daily reality.
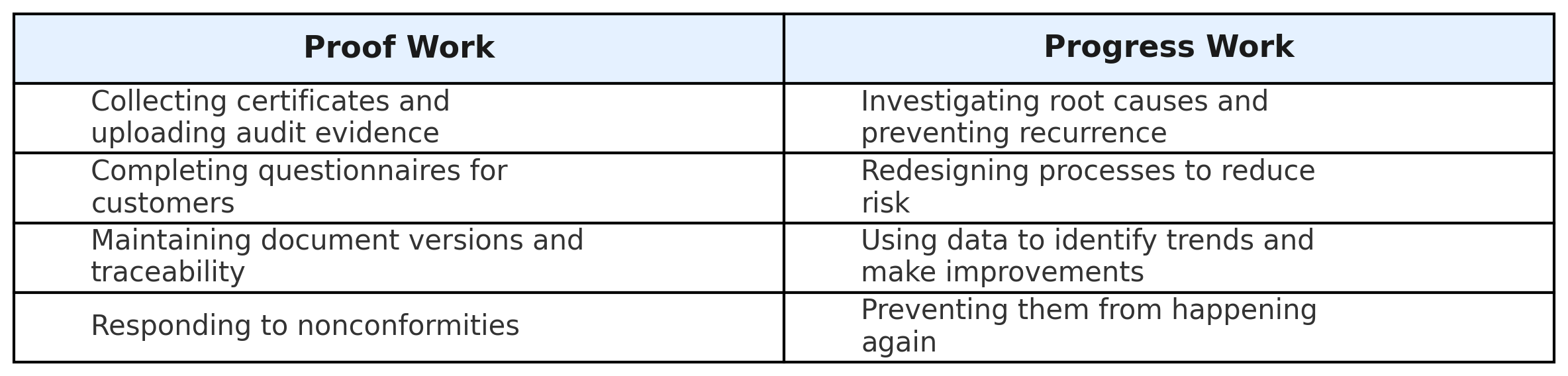
n theory, these two sides should complement each other. The proof provides structure; the progress ensures evolution. But in practice, most teams are trapped in a loop where proving compliance consumes the time needed to improve it.
A 2024 industry survey of quality and ESG professionals found that over 70% of respondents spend more time on documentation than on preventive or improvement activities. The result is a paradox: organizations appear compliant on paper, yet internally, their systems rarely advance.
For example, a food manufacturer may flawlessly maintain BRCGS or IFS certification year after year — yet still struggle with recurring product complaints, supplier issues, or waste inefficiencies. The compliance system is strong, but the learning system isn’t.
Quality has become proof-heavy and progress-light. Not because teams don’t want improvement, but because the administrative burden leaves little room for it.
The outcome?
- Preventive actions are postponed.
- Audit results repeat the same findings.
- Improvement initiatives stall under document load.
This imbalance is the hidden reason why so many quality departments feel busy — but not necessarily better.
Why Compliance Pressure Takes Over
Most quality teams don’t choose to spend their time proving compliance — they’re pushed into it by the systems, requests, and expectations around them.
Over time, the administrative side of quality becomes so dominant that it feels impossible to escape.
Here’s why that happens.
Endless External Requests
Every customer, auditor, and certification body wants proof — but in slightly different ways.
One retailer asks for “evidence of allergen control,” another for “your allergen management policy,” and a third for “how you ensure no cross-contamination.”
Different words, same question — answered from scratch each time.
In practice, quality teams end up spending hours reformatting and re-uploading information that already exists somewhere else. What should be a one-time task turns into a cycle of repetition that offers little value beyond satisfying another portal’s checklist.
This constant request culture forces quality managers into reactive mode — always responding, rarely improving.
Siloed Systems, Scattered Data
Even when data exists, it’s rarely unified. Supplier certificates live in shared drives, training records in HR systems, and environmental KPIs in sustainability dashboards.
So when an auditor asks for “evidence of supplier approval,” a simple request becomes a scavenger hunt across departments.
The bigger the organization, the worse the sprawl. Multi-site operations often maintain different folder structures, naming conventions, and file formats. By the time a team consolidates everything, the data is already outdated.
Without a central system to manage verified answers and documents, quality teams spend more time collecting data than using it.
Version Control Risks
Version control is the silent time thief of compliance. It’s not the missing document that causes problems — it’s the wrong one.
When three versions of a procedure circulate under similar names (e.g., AllergenPolicy_vFinal2_REAL.pdf), teams lose trust in their own documentation. A single upload error can trigger a nonconformity, even when the right process exists.
To prevent mistakes, quality professionals manually check timestamps, authors, and folder paths — an unsustainable process for organizations handling hundreds of documents.
Without automated tracking or metadata, version control consumes hours that could otherwise fuel preventive work.
A Reactive Compliance Culture
The deeper issue isn’t technological — it’s cultural. Most organizations reward compliance performance: completed audits, renewed certificates, and on-time submissions. Improvement initiatives, on the other hand, rarely get the same visibility or recognition.
So quality teams focus on what’s measurable — meeting deadlines, closing findings, and keeping auditors happy. Over time, improvement becomes “something we’ll get to after audit season.”
But audit season never ends.
The Result
The system rewards proof, not progress. And in that system, even the most capable quality professionals spend their days maintaining compliance — instead of building better processes.
Automation isn’t just a convenience here; it’s the only practical way to shift the balance. When data is verified once and reused many times, quality teams can finally step off the treadmill and focus on what they’re trained to do: improve.
The Cost of Staying in Proof Mode
At first, spending hours on documentation feels responsible — a way to keep control and avoid surprises during audits. But over time, the hidden costs of “proof mode” start to surface. They don’t just affect efficiency; they quietly erode the company’s ability to improve, innovate, and grow.
Here’s what it really costs when quality becomes a reporting function instead of an improvement driver.
a) The Hidden Time Drain
A mid-sized food manufacturer might handle over a dozen external audits each year — from customers, certification bodies, and regulators.
Each one requires collecting evidence that already exists elsewhere: supplier approvals, cleaning validation records, training logs, and traceability documentation.
If each audit preparation takes roughly 80–100 staff hours, that’s close to 1,200 hours annually — or nearly six months of full-time labor spent on repetitive tasks.
Now multiply that by multi-site operations or by customers with separate portals, and the number doubles. That’s time that could be spent investigating complaints, improving yield, or piloting preventive measures — instead of filling out forms.
b) Missed Opportunities for Root Cause Analysis
When reporting deadlines pile up, the first casualty is reflection.
Instead of analyzing why issues happen, teams focus on documenting that they happened — and that corrective actions were filed.
This is where real improvement stalls. Without time to explore root causes, the same problems recur — customer complaints, deviations, supplier delays — each logged neatly but never truly solved.
Example: A packaging company saw repeat customer complaints about seal integrity. Each incident was closed with a corrective action, but no pattern analysis was done. When they finally reviewed 18 months of data, the root cause traced back to a single machine design issue. Fixing it reduced complaints by 60%.
The insights were always there — but buried under paperwork.
c) Employee Fatigue and Turnover
Quality professionals rarely burn out from problem-solving. They burn out from repetition.
Highly skilled employees — often with years of technical expertise — spend much of their week renaming files, uploading documents, and answering the same questions across multiple platforms.
This isn’t just inefficient; it’s demotivating. In internal surveys across manufacturing and food companies, over 50% of quality managers report that administrative overload reduces engagement and contributes to turnover.
Retention isn’t about job titles or salaries — it’s about meaningful work. When compliance overshadows improvement, people lose sight of purpose.
d) The Risk of Static Systems
Ironically, teams that spend all their time proving compliance are often the least ready for change. When a new regulation (like CSRD or EUDR) appears, or a customer updates its requirements, these organizations struggle to adapt because their systems are designed for static documentation, not dynamic learning.
Continuous improvement requires flexibility — the ability to update, connect, and evolve data quickly. Proof-based systems can’t do that. They’re built for stability, not agility.
e) The Opportunity Cost of Progress
The most expensive loss isn’t time — it’s opportunity.
Every hour spent copying evidence or formatting reports is an hour not spent improving product quality, supplier performance, or sustainability metrics. When multiplied across teams and sites, those lost hours become the difference between being audit-ready — and being industry-leading.
How to Reclaim Time for Progress
Breaking out of proof mode doesn’t require tearing down your entire quality system — it requires rethinking how information flows through it.
The goal isn’t to reduce oversight; it’s to reduce repetition.
Here are five practical steps quality teams can take to reclaim time for actual improvement work.
1. Standardize and Centralize Your Data
The fastest way to regain control is to stop treating every audit, questionnaire, or customer request as a new project. Instead, store verified answers and documents in a shared, version-controlled repository.
When data lives in one place — with clear owners, review dates, and approval statuses — it can be reused across multiple forms, sites, and audits.
Example: A packaging supplier standardized its quality evidence library across five production sites. Instead of searching for “latest” versions of ISO certificates and allergen control plans, teams now use one verified set of documents.
Result: 65% less time spent on audit prep and zero version errors in the past year.
2. Automate Repetitive Questionnaires
If your team keeps typing the same answers — “Yes, we have an HACCP plan,” “Yes, our suppliers are GFSI certified” — automation can do that for you.
Modern quality systems (like Passionfruit) can automatically detect recurring questions, suggest verified answers, and attach the right supporting documents. Each answer is tagged with a confidence score and metadata so you can decide when to review or reuse it.
That means no more copy-pasting between customer portals, no more manually reformatting data, and no more guessing which version to upload.
Automation doesn’t replace quality expertise — it eliminates the repetitive admin that hides it.
3. Build Context Into Every Answer
Quality data without context is risky. A certificate is only valuable if you know:
- Who uploaded it
- When it was verified
- Which location it applies to
- When it expires
By embedding metadata and confidence scores into every document or answer, teams can instantly assess reliability.
When an auditor asks for proof, you don’t need to check ten folders — you can simply filter by confidence level, date, or location.
This kind of structured data turns static files into dynamic, reusable evidence.
4. Redefine Quality KPIs
If your team’s performance metrics revolve around audit completion rates or number of closed nonconformities, you’re measuring proof, not progress.
Introduce improvement-focused KPIs such as:
- Time saved on audit preparation
- Percentage of questionnaires auto-filled with verified data
- Reduction in recurring findings
- Hours allocated to preventive or improvement projects
When leadership tracks and rewards these outcomes, quality teams shift their mindset from compliance maintenance to improvement momentum.
5. Close the Learning Loop
Improvement isn’t about collecting findings — it’s about learning from them.
Establish a monthly or quarterly routine where teams review all corrective actions, identify common causes, and agree on one system-wide preventive improvement.
Document those insights in the same system where your audit data lives — not in separate spreadsheets or reports. That’s how “proof work” transforms into “progress work”: every issue becomes a learning opportunity, not another entry in a log.
Example: A food company noticed that 40% of its nonconformities were linked to supplier delays. By analyzing data across sites, they implemented shared supplier risk metrics — reducing related complaints by 25% within six months.
The Future of Quality — Proof and Progress Aligned
The role of quality has never been more complex — or more critical. Retailers, certification bodies, and regulators demand constant visibility. Customers expect transparency. And internal leadership wants faster insights, fewer risks, and proof that improvement is measurable.
In that environment, it’s easy for teams to focus entirely on documentation. But true quality isn’t about how much evidence you can upload — it’s about how much better your organization becomes because of it.
Proof and progress don’t need to compete. When systems are connected, data is structured, and repetitive work is automated, proof becomes the by-product of good processes, not a barrier to them.
The best-performing companies are already proving this shift. Their audits take less time, their improvement cycles move faster, and their teams are more engaged because they finally have the bandwidth to focus on meaningful work.
In practical terms:
- Audit readiness becomes continuous, not seasonal.
- Reuse replaces repetition.
- Learning becomes part of daily operations — not something squeezed in between reporting deadlines.
That’s the future of quality management: one where compliance is verified automatically, and improvement happens intentionally.
Where Passionfruit Fits
This is the shift Passionfruit enables.
It was designed for teams that are stuck between compliance and improvement — spending more time proving they’re compliant than improving how they work.
Passionfruit connects the dots between data, documents, and questionnaires, so quality professionals can focus on analysis instead of administration.
- Centralized, verified answers eliminate repetitive data entry across customer and certification portals.
- Confidence scoring helps teams instantly see how reliable and current each piece of information is.
- Context tagging ensures every answer includes location, owner, and version data — so no one has to double-check which file is “final.”
- Automation fills in recurring questions and updates evidence automatically, saving days of prep work for every audit.
- Integrations with Excel and Outlook let teams respond from the tools they already use, keeping workflows natural and efficient.
The result is a quality system that proves itself — without the paperwork chase. Proof happens in the background, progress happens by design.


